ISO 13485:2016 Lead Auditor Training
PAS929 Type: E-Learning

Description
To fulfill all requirements of Quality Management System for medical devices, Punyam Academy’s Online ISO 13485 lead auditor training course provides training and certification which is very useful for users to become ‘Certified ISO 13485 lead Auditor’. In this ISO 13485 lead auditor training online, you will learn everything you need to know about the QMS and ISO 13485:2016 requirements as the ISO 13485 lead auditor. This online ISO 13485 lead auditor course will help to guide company employees of any organization as well as provides a well understanding of various activities involved in Audit Techniques, Audit Processes, and Requirements. The participants will be awarded ISO 13485 Lead Auditor certificate for a trained lead auditor with successful completion of this online lead auditor training course.
TOPICS COVERED UNDER ISO 13485 LEAD AUDITOR TRAINING COURSE
There is including a total of 9 interactive online sessions this ISO 13485 lead auditor training with combinations of lectures with audio-visual presentation, hand-outs, and videos as well as online exams to ensure a complete understanding of the subject.
- Session - 1: Overview of Course and Standard
- Session - 2: ISO 13485 Principles
- Session - 3: ISO 13485:2016 Requirements
- Session - 4: Control of Documents and Records
- Session - 5: Risk Management
- Session - 6: ISO 13485:2016 Internal Audit Process
- Session - 7: Audit Terms and Definitions and Roles and Responsibilities
- Session - 8: Performing an Audit
- Session - 9: Nonconformity and Corrective Action
Each of the above session consists of modules such as Audio lectures, hand-outs, videos and session exams as well as one final exam as below:
- Lectures: There is a total of 9 lecture sessions, which are given in this ISO 13485 lead auditor course as a presentation with explanatory audio and visuals to understand the subject well.
- Hand-outs:For all the above 9 sessions, the hand-outs are given in a total of 200 pages in .pdf format. The participants of this ISO 13485 lead auditor course can download and save the hand-outs to their computer and print them or read them offline to get detailed knowledge of all the 9 topics mentioned above.
- Manual - In Session 8, a practical example of real-life quality management system for medical devices manual is given for performing document review and understanding adequacy audit findings. Such a real-life case study of a ISO 13485 manual will help participants to gain better knowledge and skill of auditing.
- Audit Checklist - The ISO 13485 Audit checklist contains a complete set of more than 400 audit questions as per ISO 13485:2016 standard requirement-wise as well as department-wise. ISO 13485:2016 audit checklists also help participants to prepare for the final audit.
- Videos: Actual videos for performing an actual audit with the real practical example of audit questions and answers. The Lead Auditor can watch and learn how to perform the audit in the organization and manage the opening and closing meetings with management employees.
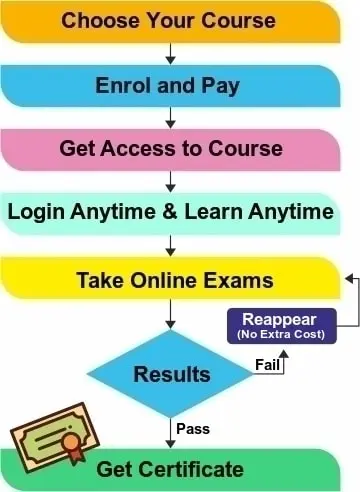
- Exams: In this ISO 13485 lead auditor training course, the participant has to clear all session exams and one final exam as given in a separate section of the course. After successfully passing the final exam, an auto-generated ISO 13485:2016 Lead Auditor Training Certificate will be issued and can be downloaded and printed from the dashboard.
COURSE OBJECTIVES
After completion of this Online course for ISO 13485 lead auditor training, the users will be able to:
- Familiarize yourself with ISO 13485:2016 requirements in detail.
- Know about the resource requirements and process requirements.
- Understand the documentation required by ISO 13485:2016.
- Understand documentation and list of procedures to be maintained, and to check them as lead auditor.
- Get the knowledge of internal auditing and use of audit checklist.
- Know about the types of auditing and questioning techniques.
- Familiarize yourself with principles & implementation of ISO 13485:2016 lead audit programs in detail.
- Get the ready-to-use audit checklist with clause-wise questions to perform an effective audit.
WHO SHOULD ATTEND THIS COURSE?
This ISO 13485 Lead Auditor Training course is developed for working professionals, management students, and other individuals for enhancing their career to new heights. Our e-learning courses help them to succeed in today's competitive environment, to renew licenses, and to update, strengthen and add quality to their existing knowledge and skills. Our courses are also useful for those who want to get a certification or start a new profession.
Any working professional, any graduate, undergraduate, management student, an entrepreneur, quality manager, technical manager, etc., are eligible to attend this certified ISO 13485 lead auditor training.
Prerequisites
- Knowledge of Quality management systems
- Understand the Plan-Do-Check-Act (PDCA) cycle.
- Knowledge of the following quality management system principles and concepts):
- The assignment of responsibility for quality;
- Incorporating management commitment and the interests of stakeholders;
- Enhancing societal values;
- Using the results of risk assessments to determine appropriate controls to reach acceptable levels of risk.
AUTHORS & INSTRUCTORS
For details of the authors, trainers and instructors experience and background, please visit our trainers page. They have very rich experience on the subject.
STUDY MATERIALS
This e-Learning course is provided with study materials and you can read it after your log in or download (PDF format). Use the study materials to reinforce key points and to keep a reminder of what you already learned as well as you can save it in your computer for future reference. The access of this study material is removed after exam is cleared and on line certificate is prepared for the student.
EXAMINATION AND COURSE CERTIFICATE
All paid E-learning course includes the Course Certificate that is issued upon course completion and passing the session exams as well as a final exam given in the course with minimum 60% marks. The option to reappear in the exam is also given to student, if failed in any exam.
END OF COURSE INSTRUCTION
After completing the session and passing each session examination the student can appear for final exam. Once the final exam is cleared then it is considered a end of course. The training certificate is ready on our LMS and student can print it or save the training certificate. It is also available for verification by entering the name and certificate number.
- Duration40 Hours (05 Days)
- Price (USD) $418
- DOWNLOAD COURSE DEMO
- TypeE-Learning
- LanguageEnglish
Search Course
Why Learn with us?

How Automated LMS Works?