ISO 35001:2019 Lead Auditor Training
PAS311 Type: E-Learning

Description
This Online ISO 35001 Lead Auditor Training Course is designed to provide you high-level training and certification to become a certified lead auditor. On successful completion of this course, you will get ISO 35001 Lead Auditor Certificate with the logo of Exemplar Global (formerly RABQSA) – a world leader in training and certification programs.
In this online lead auditor training, you will learn everything you need to know in order to conduct and lead audits of ISO 35001 biorisk management systems on behalf of any company or certification body. This online ISO 35001 Lead Auditor Training course provides complete knowledge of ISO 35001:2019 standard and the requirements for a biorisk management system. It will also equip you with the knowledge and skills of auditing process, audit techniques, nonconformity reporting as well as your roles and responsibilities as a lead auditor for biorisk management system audits. The training course comprises audio-visual presentations, handouts, ISO 35001 audit checklist, real-life case study of an ISO 35001 system manual and online exams, which will be quite beneficial for all those who want to qualify as certified Lead Auditor for ISO 35001 audit of laboratories and related organizations that works with, stores, transports, and/or disposes of hazardous biological materials, in any part of the world. The online training course is beneficial especially for those who prefer to learn auditing process and techniques from the comfort of their home or office at their own convenient time and get certified as lead auditor. After completion of this training course successfully, the participants will be awarded ISO 35001 Lead Auditor certificate as a trained lead auditor.
Topics Covered: ISO 35001 Lead Auditor Online Course
Punyam Academy’s online ISO 35001 lead auditor training course comprises following nine sessions:
- Session - 1: Overview of Biorisk Management System Standard
- Session - 2: ISO 35001 Requirements (Clause and sub-clause wise)
- Session - 3: ISO 35001 Documented Information
- Session - 4: Risk Management (includes risk assessment and risk treatment)
- Session - 5: Audit Process
- Session - 6: Audit Terms & Definitions and Roles & Responsibilities
- Session - 7: Performing an Audit
- Session - 8: Nonconformity Reporting and Corrective Action
- Session - 9: Climate Action Changes - New Amendments (2024)
Each of the above sessions consists of audio-visual presentations, handouts, and session exams. In Session 7, a practical example of a real-life management system manual is given for performing document review and understanding adequacy audit findings. Such real-life case studies of a system manual will help participants to gain better knowledge and skill of auditing.
Overall, the contents of this online course include the following:
- Lectures: In this module, the user gets an audio-visual presentation explaining each of the above-mentioned topics to understand the subject well.
- Handouts: For all the above 8 sessions, the handouts are given in total approx. 200 pages in PDF format. The participant of this lead auditor course can download and save the handouts in their computer for future reference. Also, they can print or read the contents offline to get detailed knowledge of all the topics.
- Videos: Actual video of performing an actual audit with real and practical examples of audit questions and answers. Participants can watch and learn how to perform and lead the audit of an organization and manage the opening and closing meetings with the management and personnel of the organization.
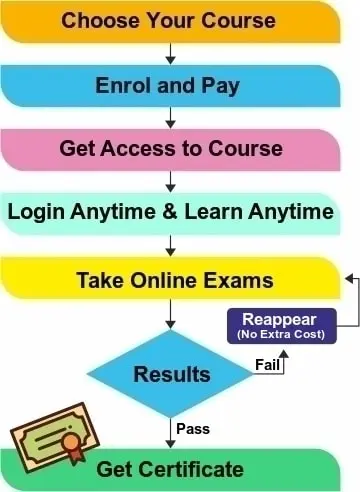
- Exam: Each session of this course contains an online session exam. Participants have to pass the exam after the completion of each session. After passing the final exam, the auto-generated ISO35001 lead auditor certificate can be downloaded and printed by the participants. This certificate can be verified by anyone anytime from this website.
- Audit Checklists: ISO 35001 clause-wise audit checklist having more than 300 audit questions are given in handouts, which can be used as a readymade audit tool. Participants can download and use the checklists for future reference during the audit of a biorisk management system.
COURSE OBJECTIVES
After completion of this Online ISO 35001 Lead Auditor Training course, the users will be able to:
- Familiarize with standard for biorisk management system, including its benefits;
- Know, in detail, the ISO 35001 requirements for biorisk management system;
- Understand biorisk management system documentation structure and documented information required by ISO 35001;
- Know about the types of auditing and questioning techniques;
- Understand how to perform and/or lead certification audits with the use of audit checklist;
- Understand the concepts of risk management, risk evaluation and treatment;
- Get confidence to conduct opening and closing meetings during any audit;
- Know about the types of nonconformities and how to close them;
- Enable yourself to lead an audit team for ISO 35001 certification audits;
- Get readymade ISO 35001 clause wise audit checklist to perform an effective audit;
- Understand details of the opening meeting as well as closing meeting and auditing techniques to perform a certification audit as a certified ISO 35001 lead auditor.
WHO SHOULD ATTEND THIS COURSE?
- Individuals, including working professionals and students, who want to become an ISO 35001 Lead Auditor having globally-recognized certificate with Exemplar Global logo.
- Individuals involved in the implementation and/or audit of biorisk management system in a laboratory or related organization.
- Individuals who want to have complete knowledge of ISO35001 standard for implementing biorisk management system with effective controls in any organization.
- Other people who have found this course useful and who want to enhance their auditing knowledge and skills, and those looking to achieve formal recognition as a trained & certified ISO 35001 lead auditor.
PREREQUISITES
Ability to understand information and instructions in English language is a must for participants. Following will be advantageous, though not pre-requisites:
- Interest in assessment/audit work
- Interest in biosafety and biosecurity issues.
- Having read ISO35001 standard
AUTHORS & INSTRUCTORS
For details of the authors, trainers and instructors experience and background, please visit our trainers page. They have very rich experience on the subject.
STUDY MATERIALS
This e-Learning course is provided with study materials and you can read it after your log in or download (PDF format). Use the study materials to reinforce key points and to keep a reminder of what you already learned as well as you can save it in your computer for future reference. The access of this study material is removed after exam is cleared and on line certificate is prepared for the student.
EXAMINATION AND COURSE CERTIFICATE
All paid E-learning course includes the Course Certificate that is issued upon course completion and passing the session exams as well as a final exam given in the course with minimum 60% marks. The option to reappear in the exam is also given to student, if failed in any exam.
END OF COURSE INSTRUCTION
After completing the session and passing each session examination the student can appear for final exam. Once the final exam is cleared then it is considered a end of course. The training certificate is ready on our LMS and student can print it or save the training certificate. It is also available for verification by entering the name and certificate number.
- Duration40 Hours (05 Days)
- Price (USD) $489
- DOWNLOAD COURSE DEMO
- TypeE-Learning
- LanguageEnglish
Search Course
Why Learn with us?

How Automated LMS Works?